Molality formula - Omni Calculator logo
Molality Formula & Examples
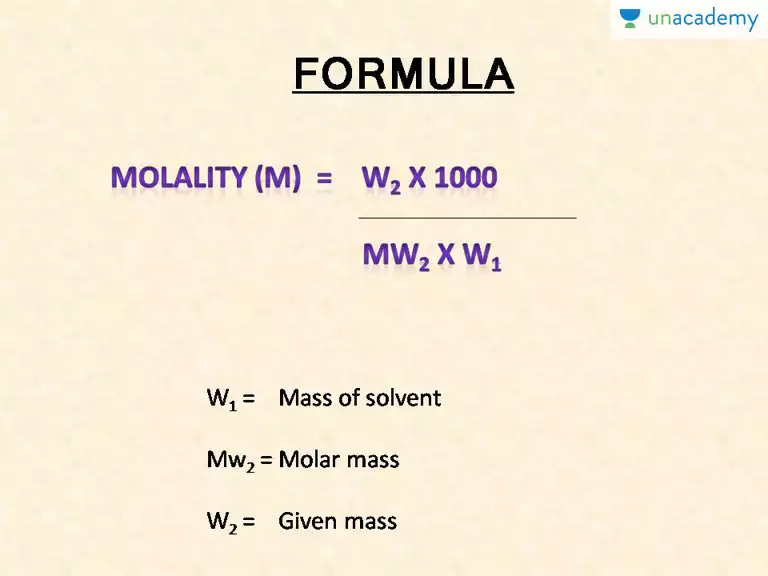
All the quantities of the solvent is represented subscript 1 e.
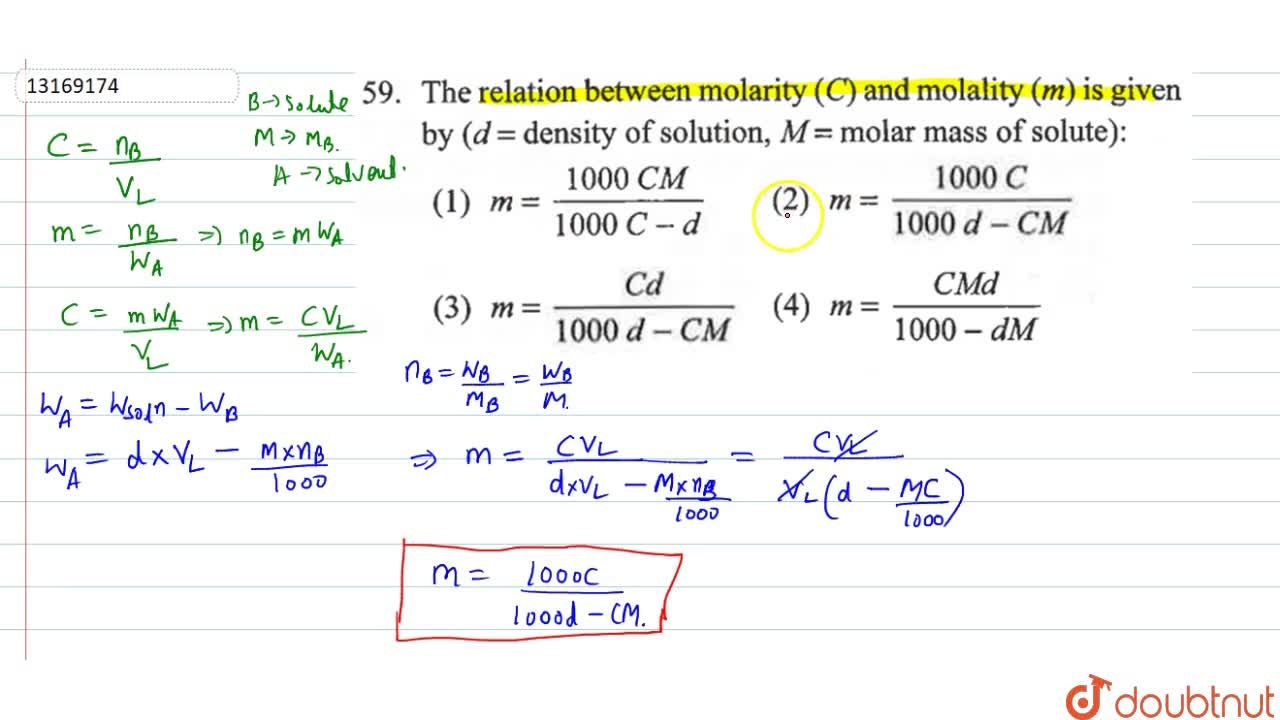
As the molal concentration of NaOH increases, the amount of NaOH also increases by the same proportion.
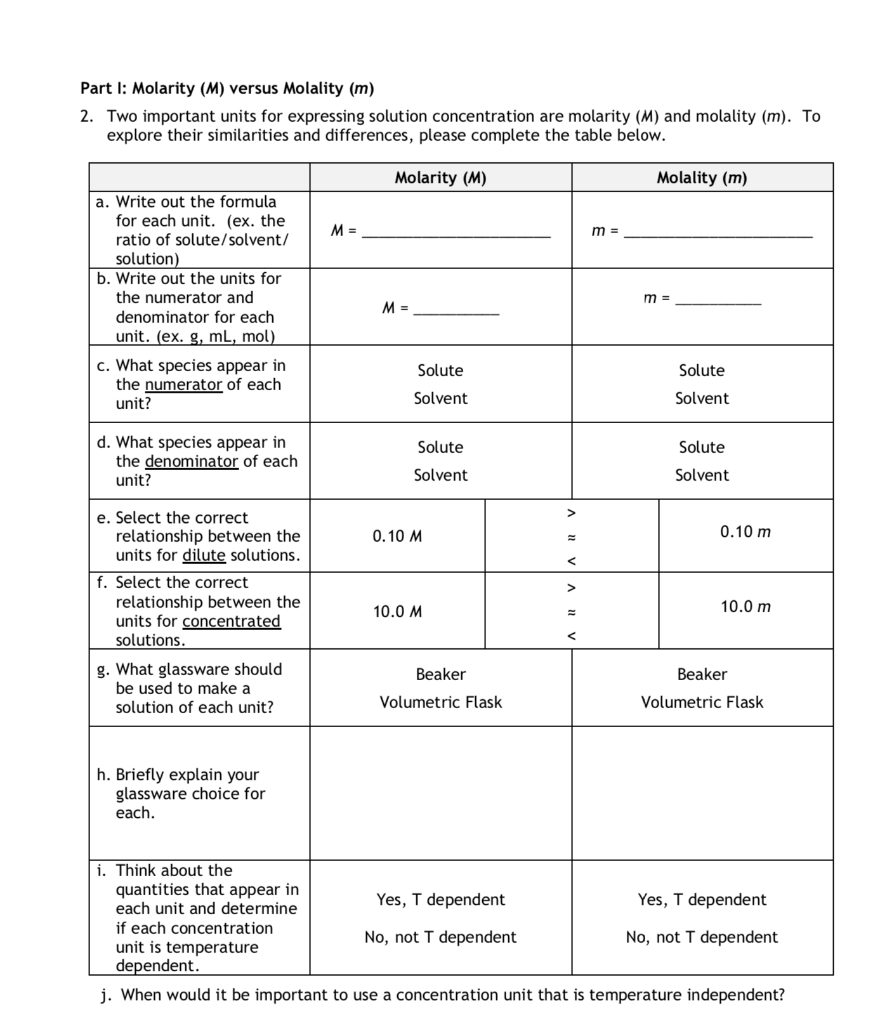
Molality Definition When we are measuring the physical parameter of a solution, what should we do? Molality differs from other solution units such as mass percent, volume percent, parts per million, and molarity.
Molality Formula, Definition and Solved Examples
The denotation is always in uppercase.
Mole fraction is divided into two, solute mole fraction and solvent mole fraction.
Let m water be the mass of the solvent.
- Related articles
2022 blog.mizukinana.jp
















)




:strip_icc():format(jpeg):watermark(kly-media-production/assets/images/watermarks/liputan6/watermark-color-landscape-new.png,45,285,0)/kly-media-production/medias/1945341/original/092280600_1519798863-20180227180501_IMG_5180-01.jpeg)







