Astrazeneca covid vaccine malaysia registration - Oxford
Registering for the Astrazeneca vaccine: A step
On 10 May, the expert committee also recommended suspending the use of both vaccines.
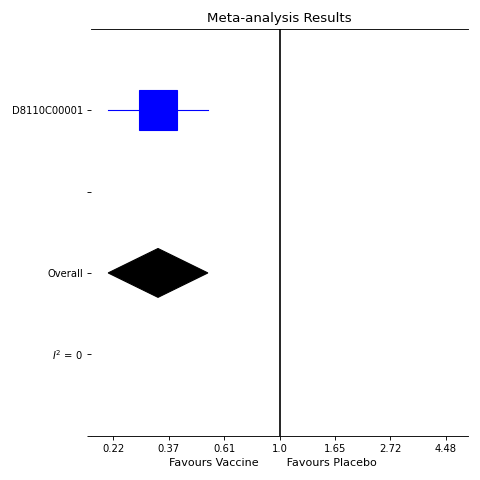
The December report showed that at 21 days after the second dose and beyond, there were no hospitalisations or severe disease in those who received the vaccine, compared to 10 cases in the control groups.
The active ingredients would be produced in Argentina and sent to Mexico to be completed for distribution.
Khairy: New round of AstraZeneca opt
.
Registration for its 268,800 slots opened at noon on May 2 and was fully snapped up within three hours.
Beginning 11 May, multiple provinces announced that they would suspend use of the AstraZeneca vaccine once again, citing either supply issues or the blood clotting risk.
- Related articles
2022 blog.mizukinana.jp