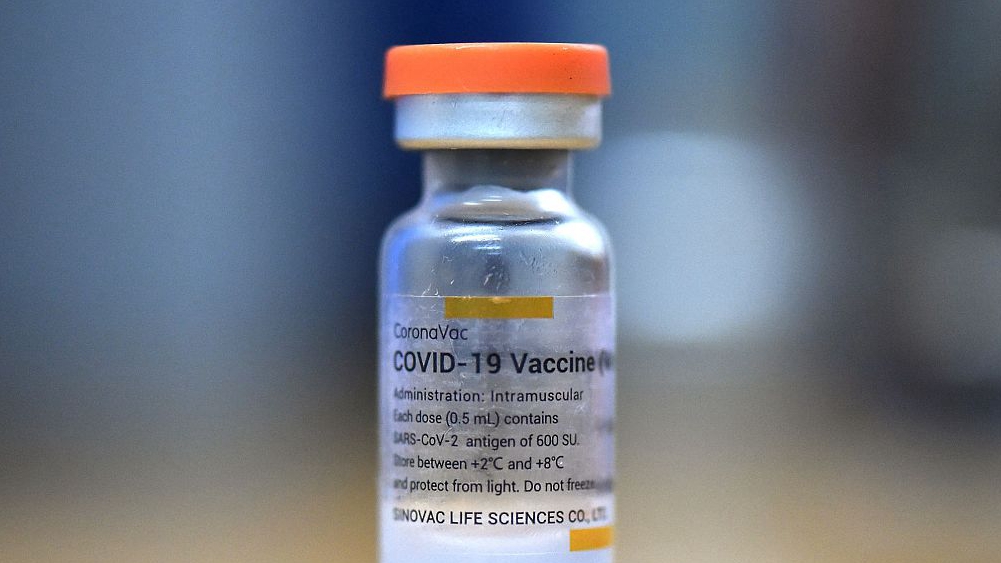Sinovac vaccine how many ml - Sinovac maker advises longer gaps between doses
List of COVID
This means that the formaldehyde content in the vaccine is 180—250 times the upper limit set by the Chinese Pharmacopoeia.
The greatest increases were seen with a heterologous booster, resulting in 96% effectiveness against hospitalization and 93% against symptomatic disease.
Both vaccines induced SARS-CoV-2-specific CD4+ and CD8+ T-cell responses at one-month post-vaccination but CoronaVac elicited significantly higher structural protein-specific CD4+ and CD8+ T-cell responses.
- Related articles
2022 blog.mizukinana.jp
![Vaccine many ml how sinovac Sinovac (CoronaVac) Vaccine many ml how sinovac [MISLEADING] Does](https://cdn.bangkokhospital.com/2021/07/IHL-ถาม-ตอบ-เรื่องวัคซีน-Sinopharm-OG-en-600x315.jpg)