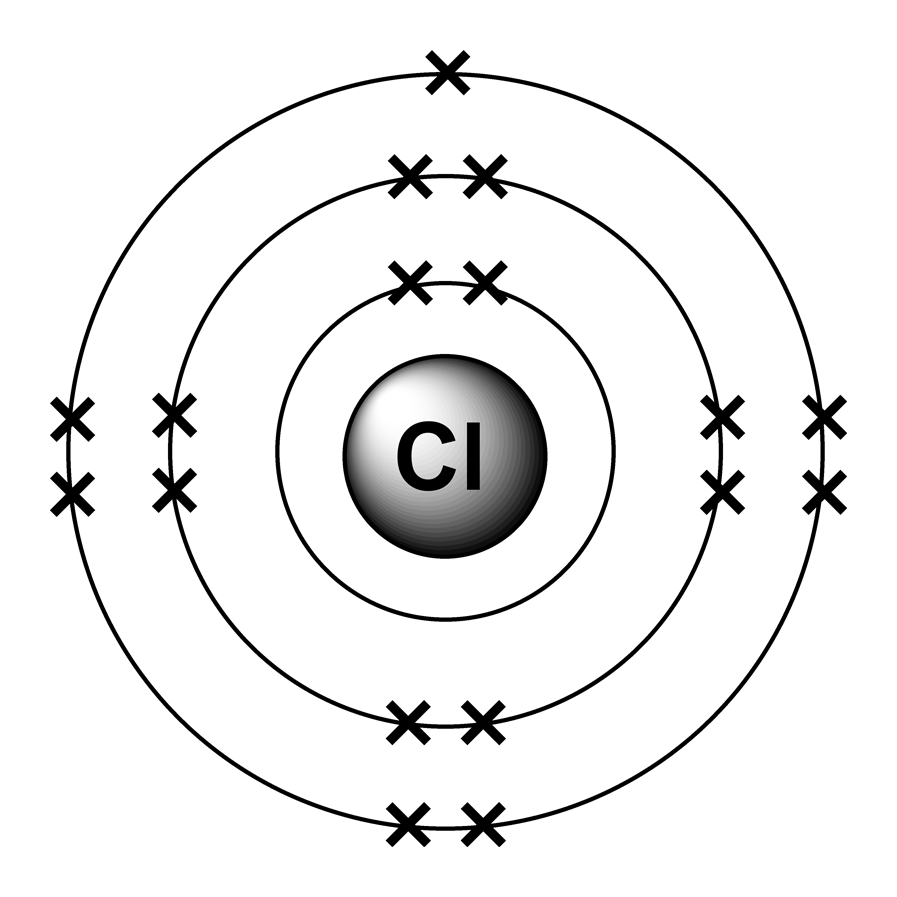
Electron arrangement - Arrangement of Electrons
Recent Posts
- Saringan covid 19 percuma selangor
- Grab paylater merchants
- Ibantuan
- Kari sotong mamak
- Bacaan oksigen kanak-kanak
- Sijil pencapaian
- Kes covid 5 februari 2022
- Selamat bersahur dan solat subuh
- Oil prices bloomberg
- Piratebay
- The roof realty penang
- Jangka tolok
- Balik kampung chord
- Resepi sambal hijau petai
- Asghar afghan
- Osc online 3.0 plus
- Yoo jae suk covid
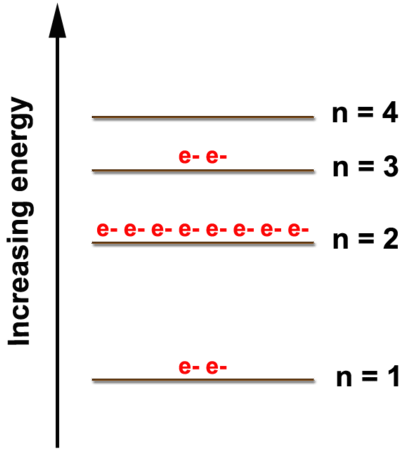
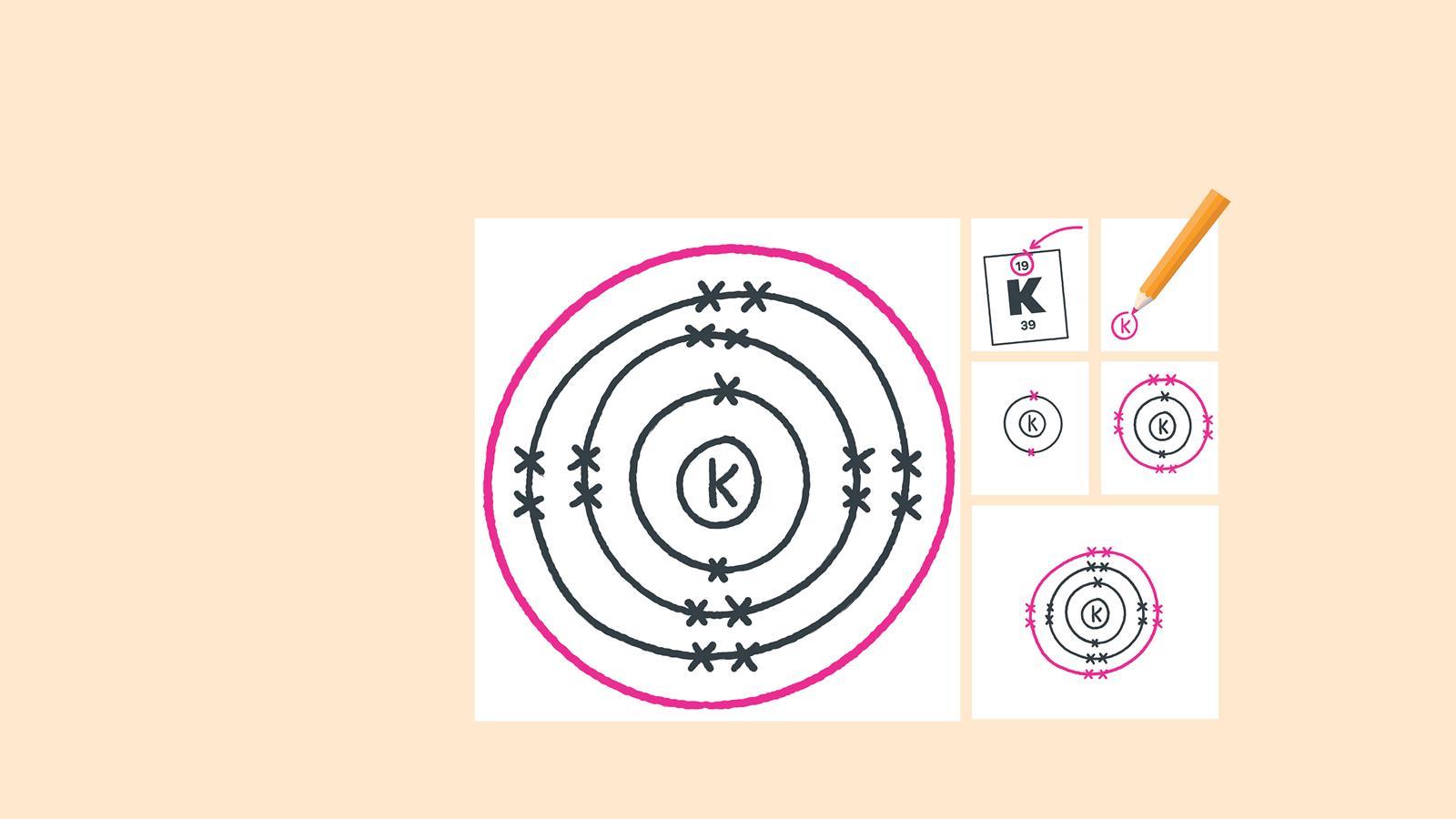
2.4: Electron Arrangements
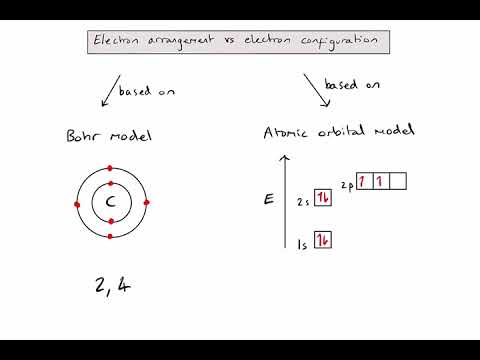
The orbital diagrams for fluorine and neon are shown.
The electrons always fill the lowest energy levels available until that level is filled, then electrons fill the next energy level until it is filled.
An atom is least stable and therefore most reactive when its valence shell is not full.
- Related articles
2022 blog.mizukinana.jp



















/cdn.vox-cdn.com/uploads/chorus_asset/file/22699631/DSCF7720_2.jpeg)