Xef4 polar or nonpolar - Is SO2Cl2 polar or nonpolar?
Recent Posts
- Honda dash 125
- Candle light dinner near me
- Partition manager
- Solat terawih sendiri perempuan
- Keputusan perdana 4d
- Faezah elai
- Cara berwudhu
- Allo wifi coverage
- Viewnet price list 2022
- Chalet darul aslah
- Pendaftaran sekolah tahun 1 2022
- Michelle yeoh guardians of the galaxy
- Kord gitar duka
- Buku teks sains tahun 5
- Honda baru 2022
- 7155 share price
- Travis and mona
Is XeF4 Polar or Nonpolar?
Only 3 of the 5 valence electrons of the phosphorus are used for bonding with chlorine, so the other two are unshared.
Polar molecules have polar covalent bonds or ionic bonds.
The atoms having higher electronegativity value attract the electrons pair from the bonding partners.
Is XeF4 Polar or Nonpolar? All You Need to Know
A carbon atom has a lower electronegative value than a fluorine atom.
Two of the electrons are shared with two chlorine atoms to form two covalent bonds.
When the vector number of these dipoles of the XeF4 molecule is computed, the resultant dipole moment is a net-zero Debye.
- Related articles
2022 blog.mizukinana.jp


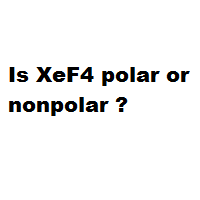



















(cropped).jpg)








